Download Lagu Temberang wallpaper. Chemistry Single Science Acids alkalis and salts.
Ph Acids And Bases Review Article Khan Academy
The greater the concentration of hydroxide ions the higher the acidity is.
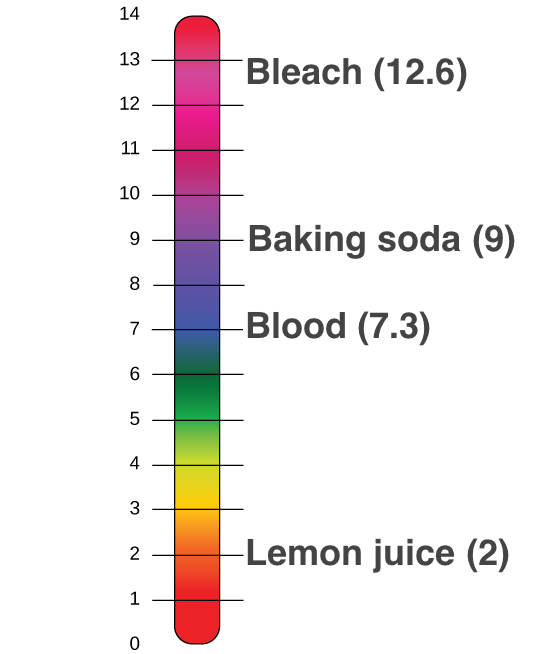
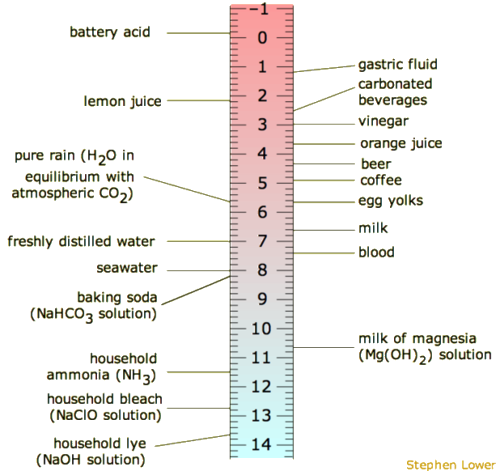
. The pH of blood ranges from 735-745 while the pH of other bodily fluids varies. The lower the pH is the more basic the solution is. The pH of a solution is a measure of its acidity or alkalinity base.
The lower the concentration of hydrogen ions the higher the acidity is. The lower the ph is the more basic the solution is. Lagu Temberang Mp3 Free Download.
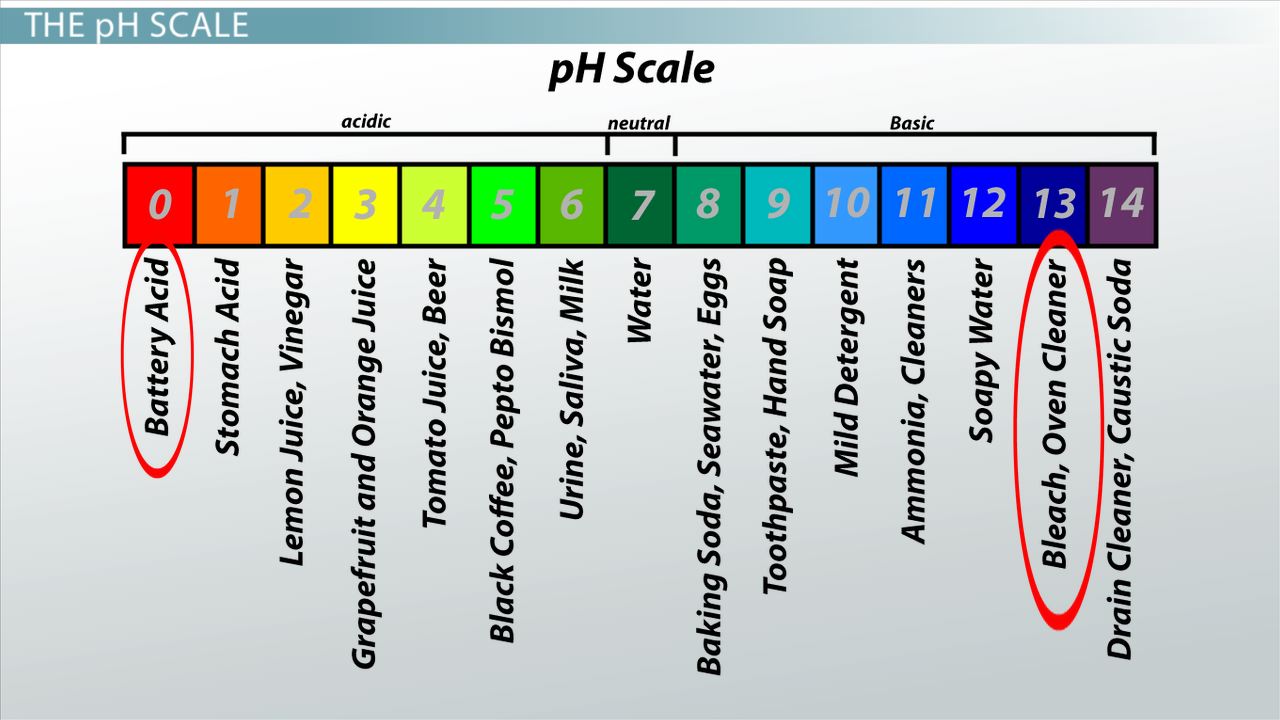
The pH scale is used to measure acidity and alkalinity. The greater the concentration of hydroxide ions the higher the acidity is. The pH scale ranges from 0 to 14.
The greater the pH is the lower the acidity is. By Da_Nathalia268 09 Apr 2022 Post a Comment Water temperature does not change rapidly. PH 0 has the highest hydrogen ion H concentration.
Describe the Relationship Between Periodic Table Group and Valence Electrons. Which of the Following Is an Example of Consideration. Test the pH of everyday liquids such as coffee spit and soap to determine whether each is acidic basic or neutral.
The pH scale ranges from 0 to 14. The lower the pH is the more basic the solution is. Investigate how adding more of a liquid or diluting with water affects pH.
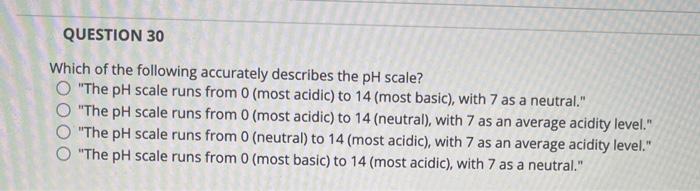
In a pH expression the hydronium ions H3O can be abbreviated simply as H. The lower the pH is the more basic the solution is. The pH scale runs from 0 most acidic to 14 most basic with 7 as a neutral.
The pH scale runs from 0 most basic to 14 most acidic with 7 as a neutral. Which of the following accurately describes the ph scale. The greater the concentration of hydroxide ions the higher the acidity is.
The greater the pH is the lower the acidity is. For each pH unit increase there is a 10-fold decrease in. A solution with a pH of 4 has twice the H of a solution with a pH of 2.
When an acid is neutralised it forms a salt. The lower the concentration of hydrogen ions the higher the acidity is. The greater the concentration of hydroxide ions the higher the acidity is.
Since the scale is based on pH values it is logarithmic meaning that a change of 1 pH unit corresponds to a ten-fold change in H ion concentration. Which of the following accurately describes the pH scale. The greater the pH is the lower the acidity is.
The greater the ph is the lower the acidity is. By Da_Nathalia268 16 Apr 2022 Post a Comment The periodic table and valence electrons worksheet answers. Question 8 1 pts Which of the following accurately describes the pH scale.
In a pH expression the hydronium ions H3O can be abbreviated simply as H. Which of the following statements about the pH scale is not true. The pH scale runs from 0 most acidic to 14 neutral with 7.
Which of the following accurately describes the pH scale. The pH of various bodily fluids on the other hand differsThe pH of saliva is between 65 and 75. The pH scale runs from 0 neutral to 14 most acidic with 7 as an average acidity level.
Correct answer to the question Which of the following accurately best describes the ph scale. The lower the concentration of hydrogen ions the higher the acidity is. Low pH suggests that there are too many H ions present while high pH indicates that there are too many OH- ions present.
3 valence electrons ex. 20 Which of the Following Accurately Describes the Ph Scale. You have probably used litmus paper paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator to test how much acid or base alkalinity exists in a.
Which of the following accurately describes the pH scale. The pH scale is often said to range from 0 to 14 and most solutions do fall within this range. The lower the concentration of hydrogen ions the higher the acidity is.
The pH scale is used to rank solutions in terms of acidity or basicity alkalinity. PH 14 has the highest hydroxide ion OH- concentration. Coma can occur if the pH falls below 69.
Which of the following is incorrect regarding the pH scale. B Scientists developed the geologic time scale. The pH would increase as the solution became more basic.
Solved Question 38 Why Is Life Based On Carbon Based Chegg Com
0 Comments